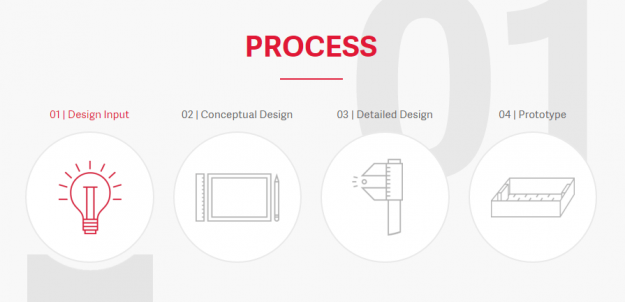
Estes’ Medical Product Development Process Aligns with Best Practices from June MPO Article
A recent Medical Product Outsourcing (MPO) article titled Orthopedic Manufacturing: A Look at the Current State of Product Development highlighted best practices in product development to allow Orthopedic OEMs to get products to market quickly, keep costs down and exceed regulatory requirements. Authors Ronald Litke and Christopher Scifert, Ph.D. noted, “Understanding user needs early in…